-
Energy migration within the 2E state of Cr3+
M. Milos, S. Kairouani, S. Rabaste and A. Hauser
Coordination Chemistry Reviews, 252 (23-24) (2008), p2540-2551


DOI:10.1016/j.ccr.2008.04.006 | unige:3575 | Article PDF
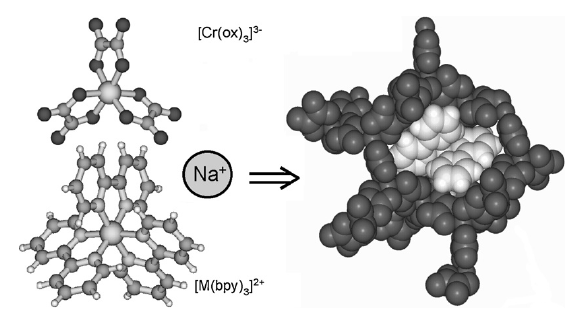
Excitation energy migration is an important phenomenon at high concentration of luminescent chromophores. In crystalline solids it results in a quenching of the intrinsic luminescence of the chromophore as the excitation energy migrates to impurity centres or other forms of trap sites. As concluded from the extensively studied systems where Cr3+ is doped as the active chromophore into inert host lattices, energy migration in crystalline solids is usually a phonon-assisted process, in which the simultaneous creation or annihilation of phonons helps to bridge the energy miss-match in the energy levels of two neighbouring chromophores within a inhomogeneously broadened absorption band. However, in the three-dimensional network systems [Ru(bpy)3][NaCr(ox)3] and [Rh(bpy)3][NaCr(ox)3]ClO4, it proved possible to unambiguously identify three different mechanisms for energy migration within the R1 line of the 4A2 → 2E transition of Cr3+. In addition to the common temperature dependant phonon-assisted process, a resonant process between the zero-field split components of the 4A2 ground state leading to a multi-line pattern in a fluorescence line narrowing spectrum and a quasi-resonant process within the same component leading to fast spectral diffusion can be identified at very low temperature. The parameters governing these processes are discussed and the behaviour of the model systems is compared to more conventional doped oxides and related systems.